|
Hooman Fatoorehchi Assistant Professor School of Chemical Engineering University of Tehran, Tehran, Iran Formulas in this page are typed in $\LaTeX$. The McCabe-Thiele Method for Binary Distillation The Rectifying (Top) Section Operating Line The rectifying section operating line passes through the points $\left( x_D, x_D \right)$ and $\left( 0, \frac{x_D}{R+1} \right)$ and its equation reads: $ y= \frac{R}{R+1}x+\frac{x_D}{R+1}$ where $x_D$ is the molar fraction of the lighter component in the distillate and $R$ is the molar reflux ratio. The Feed Condition (q) Line The feed condition line or the q-line passes through the point $\left( z_F, z_F \right)$ and has a slope of $\frac{q}{q-1}$. So, its equation reads: $ y= \frac{q}{q-1}x-\frac{z_F}{q-1}$ where $z_F$ is the molar fraction of the lighter component in the feed stream and $q$ is the mole fraction of liquid in the feed. Therefore, for a saturated liquid feed we have $q=1$, and for a saturated vapor feed, $q=0$. Of course, for a saturated liquid and vapor feed, $0\lt q\lt 1$. The Stripping (Bottom) Section Operating Line The stripping section operating line passes through the point $\left( x_W, x_W \right)$ and the intersection of the q-line and the rectifying section operating line. By $x_W$, we mean the mole fraction of the lighter component in the bottom product of the column. From the following expository figure, we can assert that our distillation column has more than 5 and less than 6 theoretical stages (approximately 5.8). 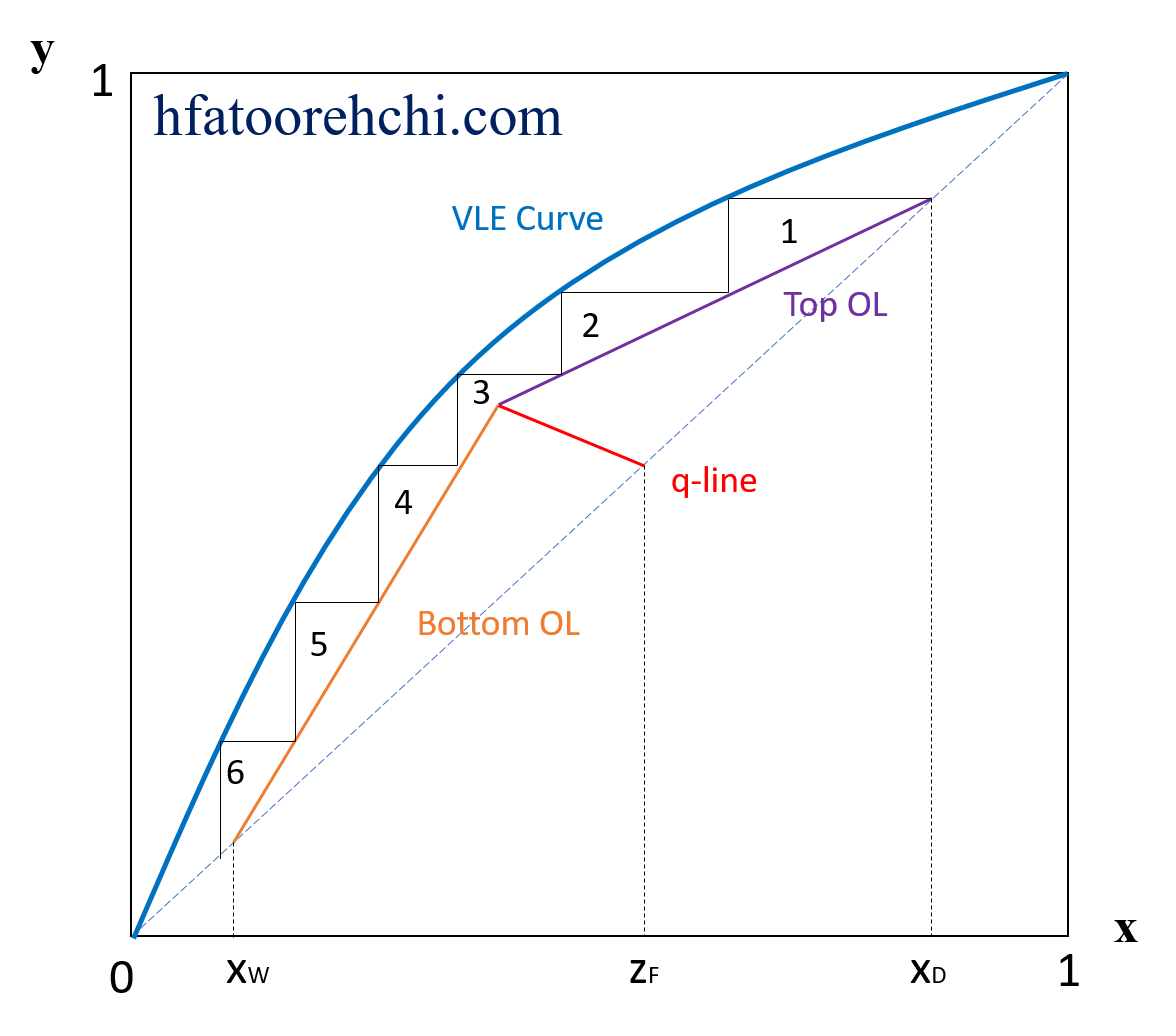
Note that the previous figure (McCabe-Thiele diagram) is drawn for a constant pressure (column's average pressure, which is constant). If we assume a constant (average along the column height) relative volatility $\alpha$, the vapor liquid equilibrium (VLE) curve will obey the following relationship: $ y= \frac{\alpha x}{1+ (\alpha-1)x}.$ Once Raoult's law is valid, i.e. the vapor and liquid phases are both ideal, the relative volatility can be calculated by $ \alpha=\alpha_{LC, HC} = \frac{P^{sat}_{LC}}{P^{sat}_{HC}}$, where subscripts LC and HC denote the light component and the heavy component, respectively. The vapor pressures, $P^{sat}_{LC}$ and $P^{sat}_{HC}$, can be obtained from Antoine's empirical equation for each component. Solved Examples T-x-y diagram Batch (Rayleigh) distillation McCabe-Thiele distillation (1) McCabe-Thiele distillation (2) Liquid-Liquid Extraction |
